Which Elements Do Not Follow the Octet Rule
Chlorine and sulfur will not strictly follow the octet rule. Not yet answered 1 Points possible.

7 3 Lewis Symbols And Structures Chemistry
Hydrogen Fluorine Sulfur Carbon Chlorine Oxygen Question 32 Status.
. On the other hand some elements exhibit hypervalency and have the ability to form hypervalent molecules. Answer 1 of 2. Its usually obeyed by the main group elements.
100 Which elements do not strictly follow the octet rule when they appear in the Lewis structure of a molecule. These elements always have one electron pair less than normal configuration. This rule is not strictly followed by some elements such as sulphur and chlorine.
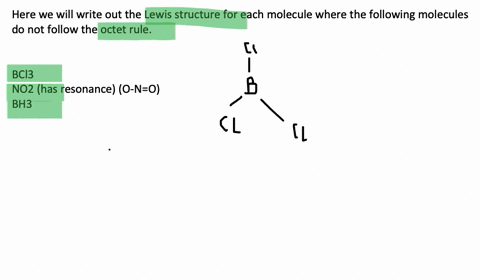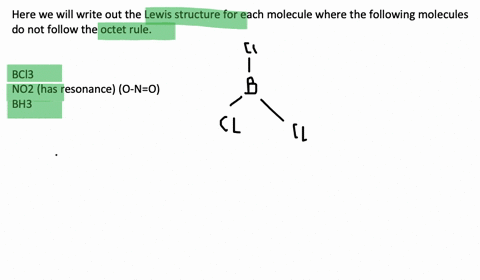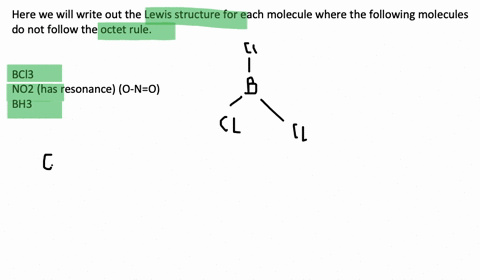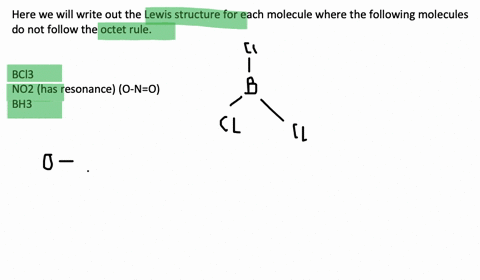
An octet corresponds to an electron configuration ending with s2p6. Chlorine and sulfur will not strictly follow the octet rule. The only possibility for boron is to bond to three hydrogen atoms in which case it forms a compound borane BH3 that does not fulfill the octet rule.
The atoms of these elements can sometimes expand their octet by utilizing the d-orbitals found in the third principal energy level and beyond. So we need 2 more covalent bonds to form an octet. The octet rule states that for atoms to be stable they must have eight electrons on their outermost shells.
Some elements that disobey the octet rule include. I know I can eliminate E because the total. The molecule has an odd number of valence electrons.
I think two elements that does not follow the octet rule are boron and beryllium. Hydrogen Fluorine Sulfur Carbon Chlorine Oxygen Question 32 Status. Not yet answered I Points possible.
Which one of the following compounds does not follow the octet rule. Elements like hydrogen lithium helium do not obey the octet rule. Select one or more.
The two elements that most commonly fail to complete an octet are boron and aluminum. So O2 does not satisfy the octet rule because as we know octet rule states that an atom has to have 8 e- in the outer shell. Molecules in which one or much more atoms possess more 보다 eight electrons such as SF6.
Calculating the formal charge on each. As the octet rule requires eight electrons around each atom a molecule with an odd number of electrons must disobey the octet rule. The central atom is in bold.
Boron is an important element for our. The octet rule states that atoms below atomic number 20 tend to combine so that they. The octet rule is described.
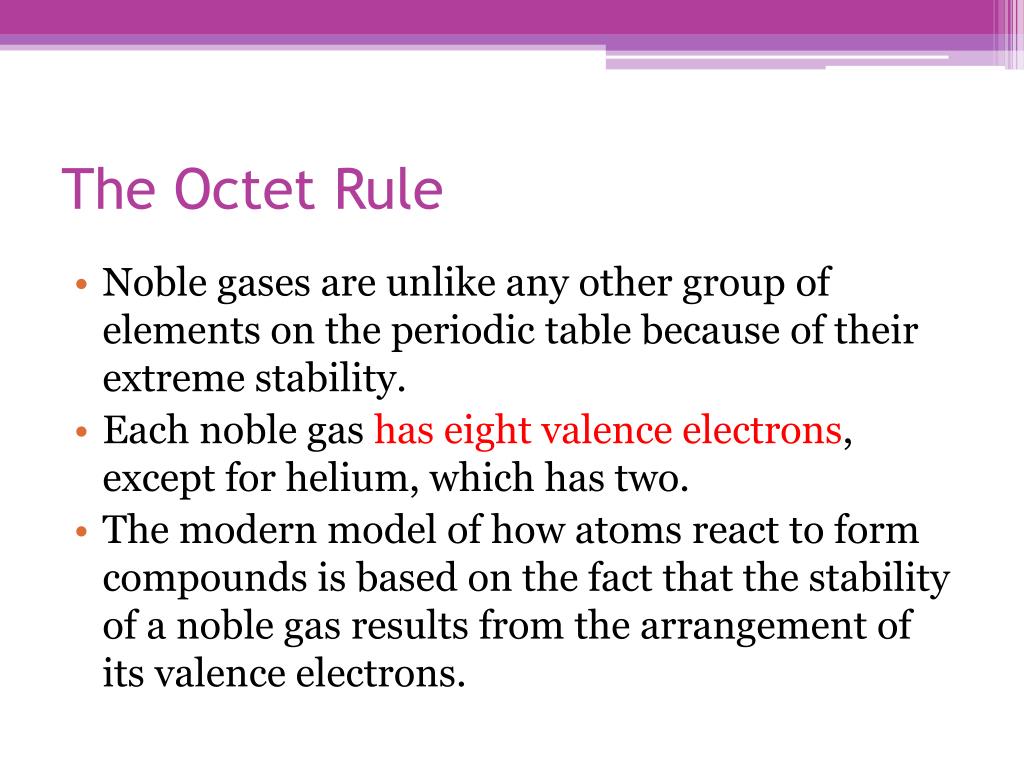
Key Takeaways The Octet Rule and Its Exceptions. The octet rule states that atoms tend to form compounds in ways that give them eight valence electrons and thus the electron configuration of a noble gas. List Four Elements that do not Obey the Octet Rule.
Explaination Expanded octet can be seen in atoms that have vacant orbital this is an essential requireme View the full answer. There are also a. Hydrogen beryllium and boron have too few electrons to form an octet.
Molecules such together NO through an odd number of electrons. Draw the Lewis structures for the following molecules or ions for which the octet rule is not obeyed. And also Molecules such as BCl3 in i beg your pardon one or an ext atoms possess less 보다 eight electrons.
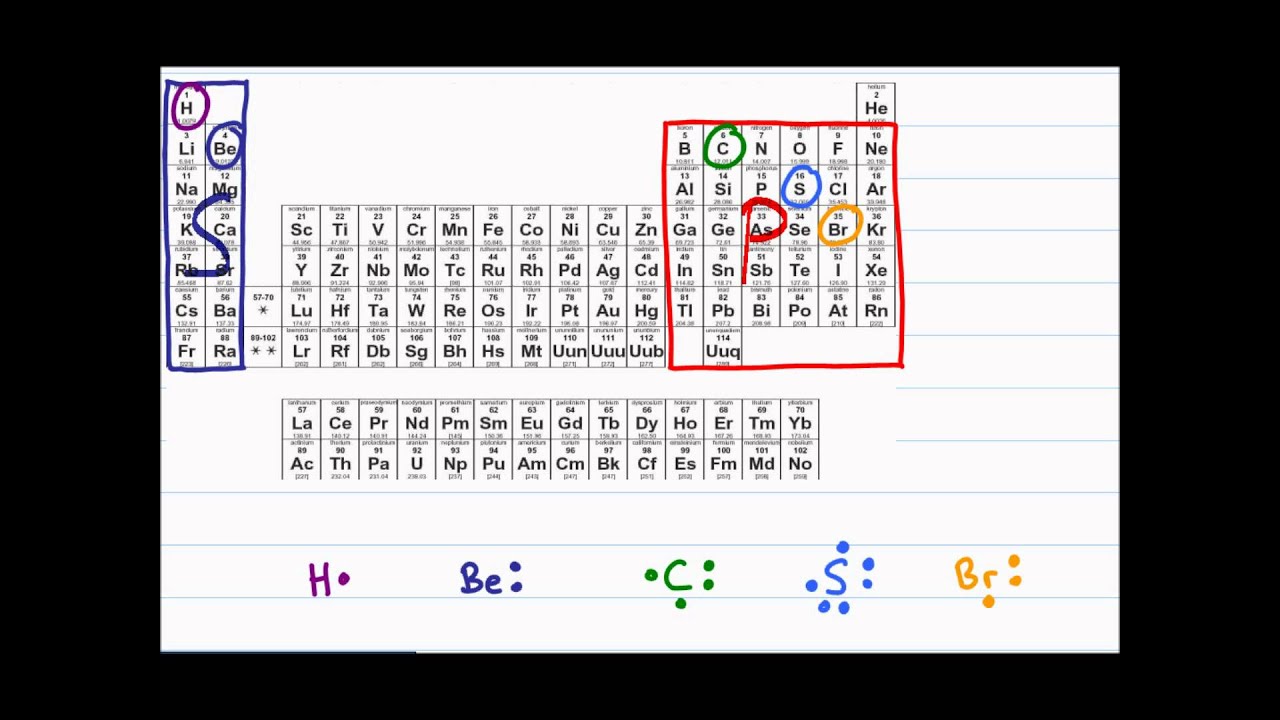
Boron has three valence electrons. Since 1p subshells do not exist some elements find stability in 1s 2 configurations. They can only lose or gain one electron to become stable due to which they follow the octet rule.
Elements like hydrogen lithium helium do not obey the octet rule. Hydrogen has only one valence electron and only one place to form a bond with another atom. 100 In the Water of Hydration lab what is the.
The compound actually exists but it is highly reactive that is to say unstable. Molecules through an odd number of electrons are fairly rare in the s and also p. There are many compounds that do not follow octet rulethe rule that suggest that every element gets stability by acquiring eight electrons in outermost or valance shell but here I will quote the example of PCl5phosphorous penta chlorideIn PCl5 P atom has 5 electrons in ou.
Elements that do not follow the octet rule. Dodecaborane with 6-coordianted boron. Beryllium has only two valence atoms and can form only electron pair bonds in two locations.
7 3 Lewis Symbols And Structures Chemistry However many atoms below atomic number 20 often form compounds that do not follow the octet rule. Oxygen has 6 valence electrons the bonds should be 8-62 bonds. Sulphur has 6 valance electrons and 6 fluorine atoms also donate 6 electrons.
What element does not follow the octet rule. Key Takeaways The Octet Rule and Its Exceptions. Hydrogen beryllium and boron have too few electrons to form an octet.
The octet rule states that atoms below atomic number 20 tend to combine so that they. However there room three basic exceptions come the octet rule. Another exception of the octet rule is transition elements.
A NF3 b CF4 c SF4 d PH3 e HCl I know I can eliminate A and B because nitrogen and carbon follow the octet rule. Does BF3 have a complete octet. However many atoms below atomic number 20 often form compounds that do not follow the octet rule.
Compounds following the octet rule must have 8 electrons in the valence shell of their central atom.
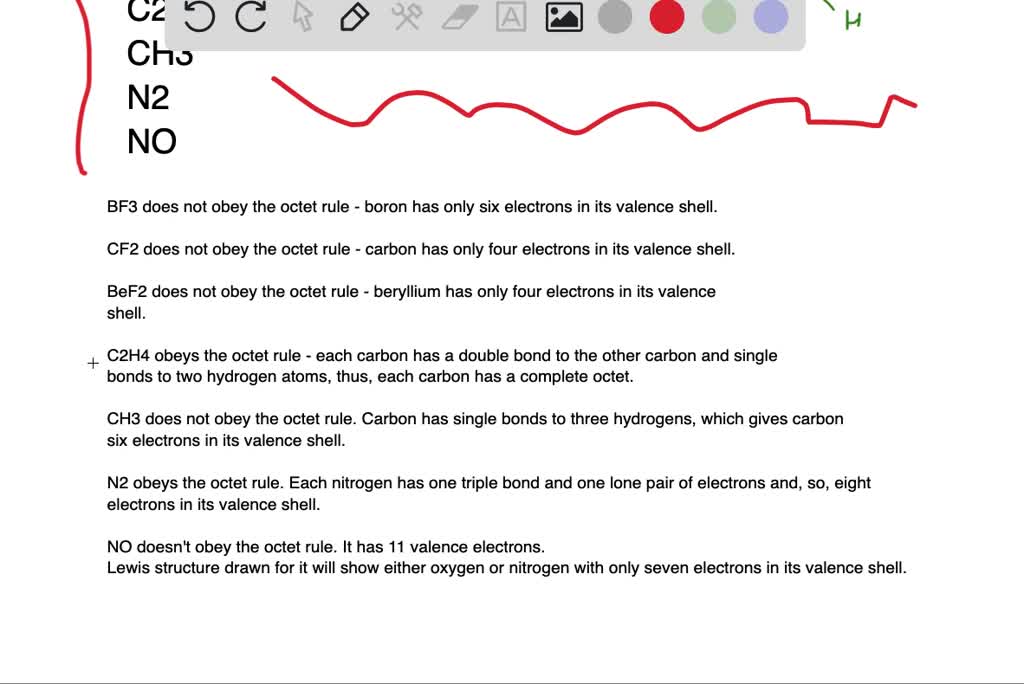
Solved Which Of The Following Molecules Have An Atom That Does Not Obey The Octet Rule Not All Of These Are Stable Molecules A B F 3 B Mathrm Cf 2 C Operatorname Be Mathrm F 2 D Mathrm C 2
Chapter 8 Basic Concepts Of Chemical Bonding Jennie

Octet Rule Duet Rule Electronic Configuration Of Lewis Electronic Structure Outermost Shell Youtube
Lewis Dot Diagram And Octet Rule Organic Chemistry Tutorial Video

Exceptions To The Octet Rule Video Khan Academy

Which Of The Following Does Not Follow The Octet Rule

Which Of The Following Compounds Does Not Follow The Octet Rule Youtube

Which Of The Following Compounds Does Not Follow The Octet Rule Youtube
8 1 Lewis Symbols And The Octet Rule Chemistry Libretexts
Ppt The Octet Rule Powerpoint Presentation Free Download Id 2654863

Solved Give Three Examples Of Compounds That Do Not Satisfy The Octet Rule Write A Lewis Structure For Each
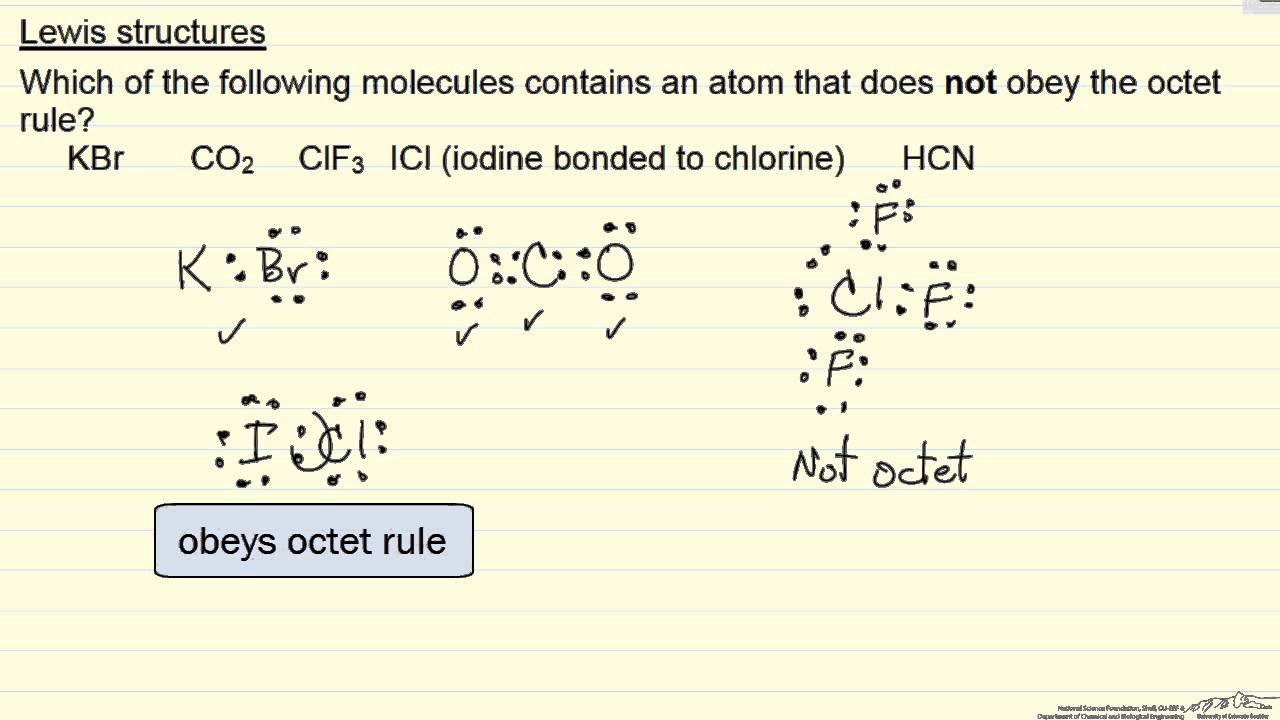
Lewis Structures Octet Rule Example Youtube
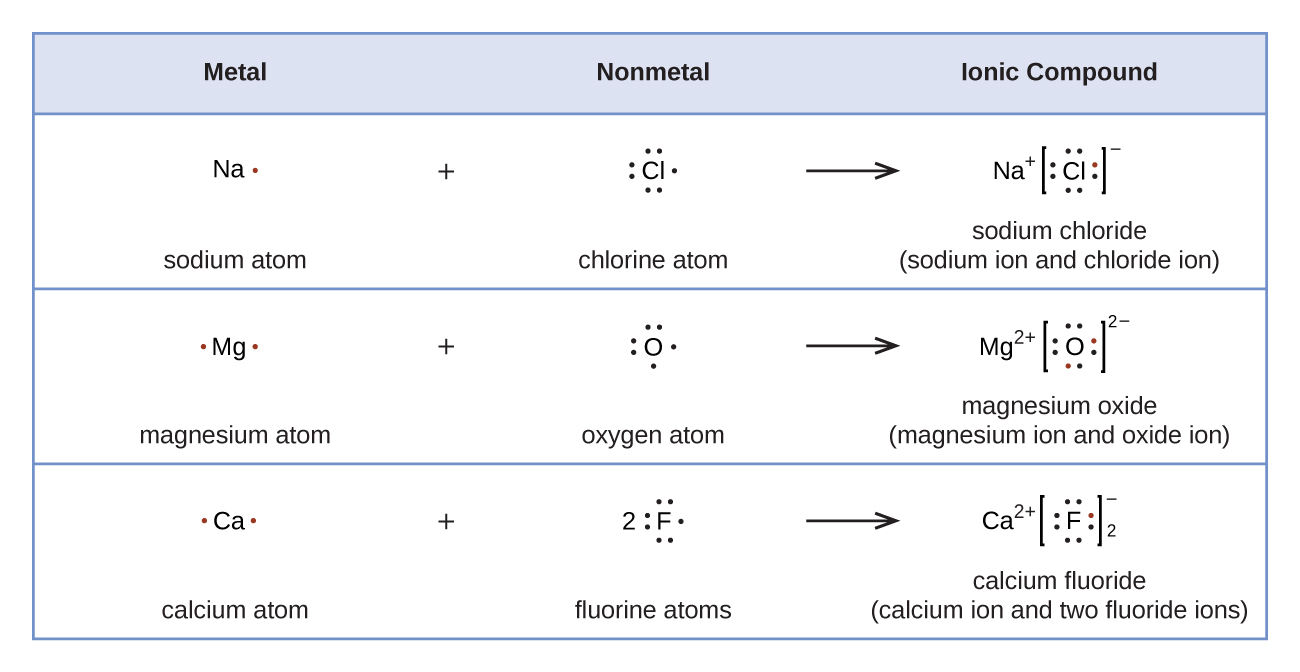
10 1 Lewis Structures And The Octet Rule Chemistry Libretexts
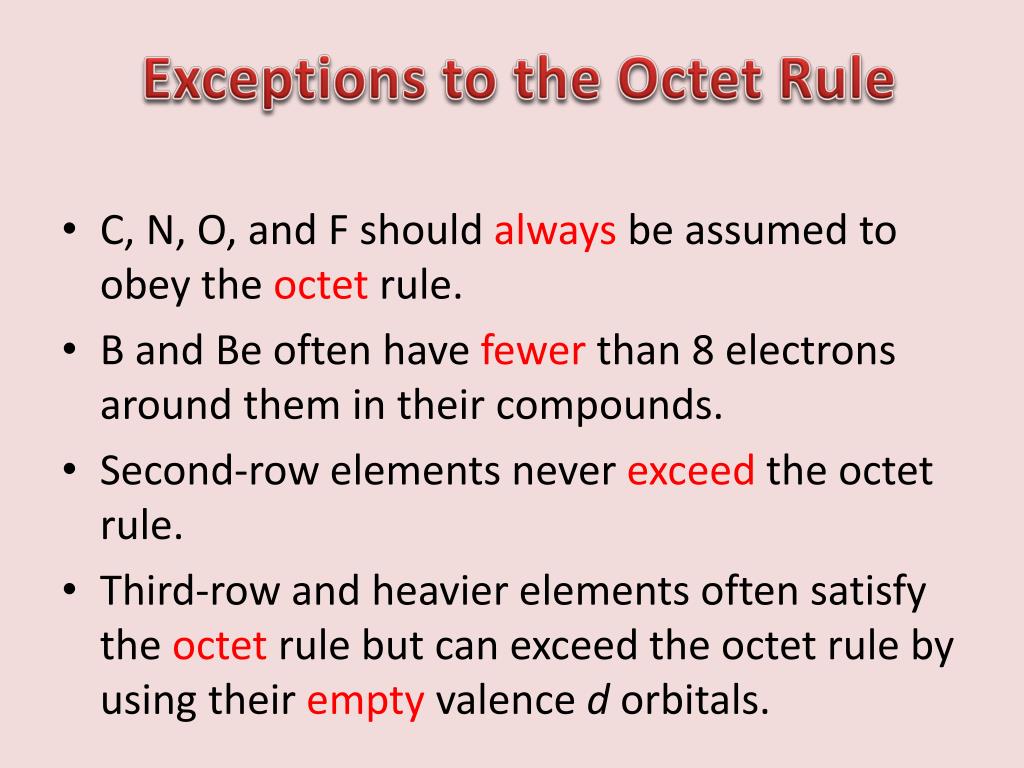
Ppt Exceptions To The Octet Rule Powerpoint Presentation Free Download Id 5318726

What Are The Exceptions Of The Octet Rule

Which One Of The Following Does Not Follow Octet Rule
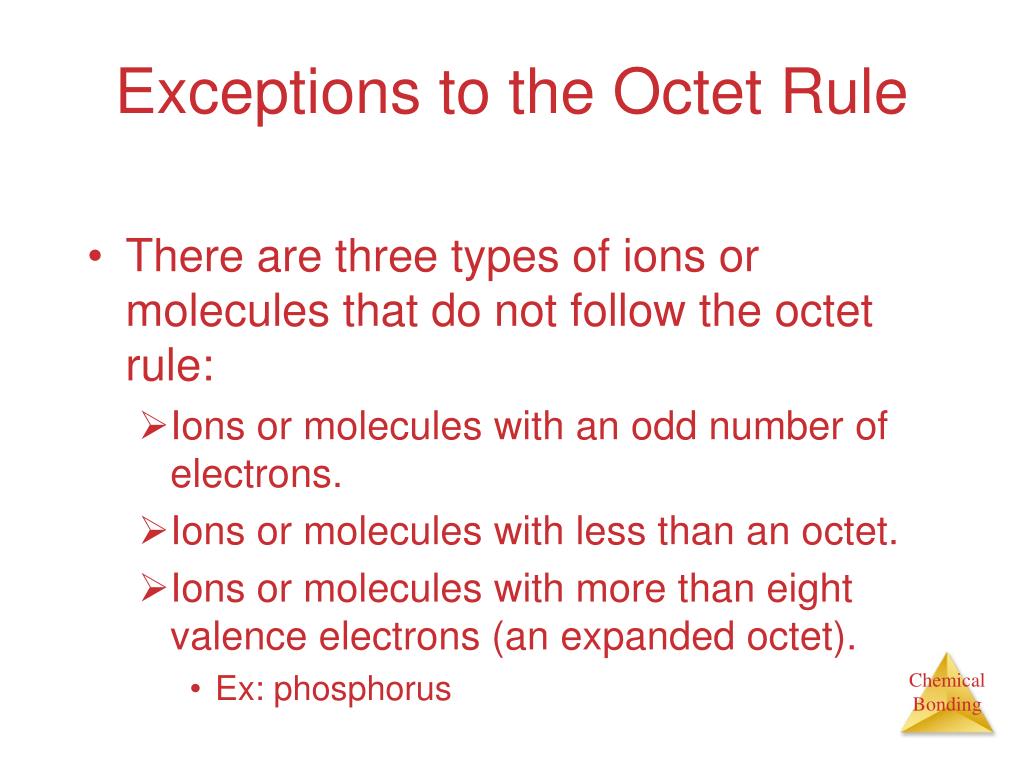
Ppt Exceptions To The Octet Rule Powerpoint Presentation Free Download Id 3528141


Comments
Post a Comment